Highlights
- Imugene Limited’s Phase 1 MAST study has cleared cohort 2 of both the intravenous (IV) and intratumoral (IT) arms of the monotherapy trial.
- The company will now commence cohort 1 of the combination study (with Pembrolizumab) and cohort 3 for both arms of the monotherapy dose escalation.
- As per IMU, early data from patients dosed at low levels with CF33 oncolytic virus reflects immune activation in the tumour microenvironment.
Clinical-stage immuno-oncology company Imugene Limited (ASX:IMU) shares rose more than 8.9% just after the market opened on Thursday following an ASX announcement by the company. IMU shares were spotted trading at AU$0.160 per share at 11:18 AM AEDT on 02 February 2023.
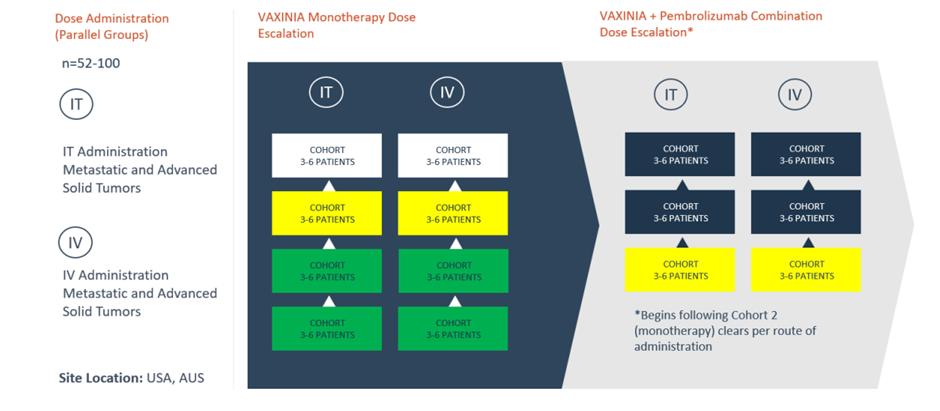
Imugene announced that its Phase 1 MAST (metastatic advanced solid tumours) clinical trial has cleared cohort 2 of both the intravenous (IV) and intratumoral (IT) arms of the monotherapy trial. This has led way for the trial to kick start cohort 1 of the combination study (with Pembrolizumab) and cohort 3 for both arms of the monotherapy dose escalation.
The Phase 1 MAST study is being undertaken with the objective to measure the safety of novel cancer-killing virus CF33- hNIS (VAXINIA).
Source: IMU update
Know more about the multicenter Phase 1 MAST trial
The clinical trial is titled “A Phase I, Dose Escalation Safety and Tolerability Study of VAXINIA (CF33- hNIS), Administered Intratumorally or Intravenously as a Monotherapy or in Combination with Pembrolizumab in Adult Patients with Metastatic or Advanced Solid Tumours (MAST).”
Imugene initiated the Phase 1 MAST study by administering a low dose of VAXINIA to enrolled subjects having metastatic or advanced solid tumours with minimum two prior lines of standard of care treatment. In preclinical laboratory and animal models, the City of Hope-developed oncolytic virus has been able to shrink ovarian, colon, lung, breast, and pancreatic cancer tumours.
To date, all those who have been treated in the monotherapy group have been given the lowest doses of VAXINIA. The patients have shown acceptable safety till now. This allows new study participants to get it in combination with the immunotherapy pembrolizumab.
Imugene started the study in May 2022. It is expected that the trial will be completed in about two years. Up to 100 patients will be recruited for the study across approximately 10 trial sites in the United States and Australia.
Management commentary

Source: Company website