Dimerix Limited (ASX:DXB) is a clinical-stage biopharmaceutical company. It develops innovative therapies for medical needs that have not been met with. DXB caters to global markets. Presently, the company is developing its proprietary product, DMX-200 for Diabetic Kidney Disease and Focal Segmental Glomerulosclerosis. DMX-200 was identified through Dimerixâ proprietary assay, Receptor Heteromer Investigation Technology, which is a scalable and globally applicable technology platform. It helps to understand receptor interactions and rapidly screen them, as well as to identify new drug opportunities.
The company was establised in 2004 - Dimerix Limited (ASX:DXB) and Dimerix Biosciences Pty Ltd (which is a 100% subsidiary of DXB) are its entities.
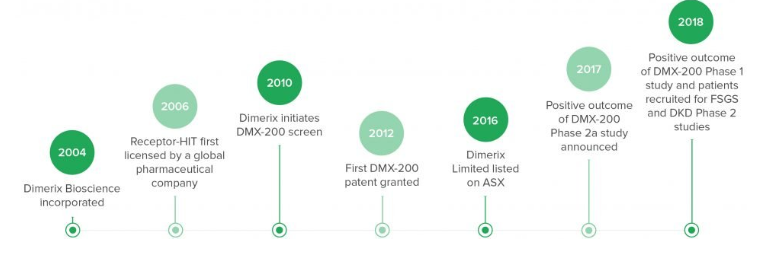 Companyâs corporate history (Source: Company website)
Companyâs corporate history (Source: Company website)
On 27th May 2019, the company provided an update on its activity related to the commercial scale manufacturing of DMX-200. The manufacturing capabilities are secured with an FDA registered manufacturer, and the demonstration batch manufacture is completed. The analytical methods had been developed and confirmed. The commercial scale batch manufacture has been planned for next year, FY2020. Furthermore, in the last quarter, this development of DXBâs manufacturing capabilities has notably progressed.
The establishment of the commercial manufacturing process and the development of validated analytical methods for DMX-200 is a vital component of DXBâs product development program. It would aid global marketing authorisations (including US FDA), commercialisation and various partnering activities.
DMX-200 is a chemical compound known by the name propagermanium. This compound restricts the activity of cellular receptor of inflammation: CCR2 (C-C Chemokine Receptor Type 2). DMX-200 is being developed by the company to cure Diabetic Kidney Disease and for Focal Segmental Glomerulosclerosis. However, it has never been approved by the FDA. It is not available as a generic drug and is not available by prescription outside Japan.
It is considered as a New Chemical Entity (NCE) by the FDA and would require a full new drug application (NDA) known as a 505(b)(1) application. The NDA should be detailed and inclusive about information like ingredients, manufacturing processes, pre-clinical study results, results in human through clinical trials, impact on the body and its packaging details.
The Commercial scale manufacture and product packaging are a part of the product development process. This can delay the marketing authorisation as stability testing of the end product is required conducted and completed in real time.
Once Dimerix undergoes the requisite FDA approved processes, it can ensure that the appropriate stability and shelf-life is known at the time of providing the NDA. This would not hold up the marketing authorisation process. The packaging would be an add on to any potential partner licencing transaction.
It is believed that DMX-200 would be the sole pharmaceutical grade and FDA approved medicine provided to the patient at the approved dose. The company owns the intellectual property and know-how of the manufacturing processes and methodology, and the demonstration batch manufacturing had already been completed.
Due to the complexity surrounding the quality of the manufacturing process and the chance of poorly manufactured propagermanium containing toxic impurities, the FDA has imposed a ban on importing any product that contains germanium into the US.
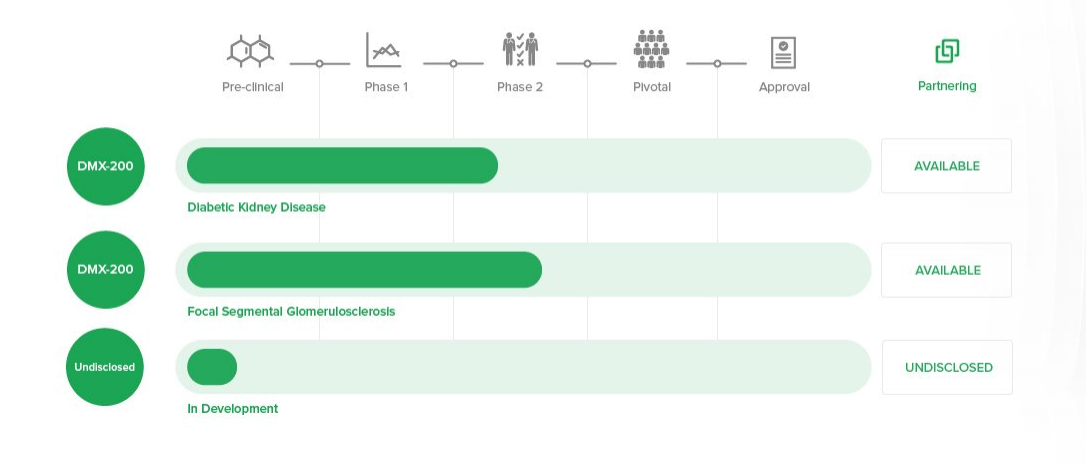 Product pipeline (Source: Companyâs report)
Product pipeline (Source: Companyâs report)
Share Price Information:
The shares of DXB are currently trading on the ASX flat at A$0.075 (as at AEST: 12:17 PM, as on 28 May2019). The market capitalisation of the company is A$11.91 million.
Disclaimer
This website is a service of Kalkine Media Pty. Ltd. A.C.N. 629 651 672. The website has been prepared for informational purposes only and is not intended to be used as a complete source of information on any particular company. Kalkine Media does not in any way endorse or recommend individuals, products or services that may be discussed on this site. Our publications are NOT a solicitation or recommendation to buy, sell or hold. We are neither licensed nor qualified to provide investment advice.






