Market Overview:
Sickle Cell Disease, a chronic genetic blood disorder, has seen rising attention due to its impact on both pediatric and adult populations. The treatment landscape is evolving with the emergence of novel curative therapies, including gene editing technologies and targeted biologics. The market is expected to expand significantly as healthcare systems emphasize early diagnosis and effective management.
Download Sample Report Here: https://www.datamintelligence.com/download-sample/sickle-cell-disease-treatment-market
Market Drivers & Opportunities:
Rising Global Disease Burden: Increased incidence rates across Africa, the Middle East, and parts of Asia and the Americas are driving demand for effective treatments.
Advancements in Gene Therapy: New curative approaches such as CRISPR and lentiviral vector-based gene therapies are transforming patient outcomes.
Government Support & Orphan Drug Designation: Regulatory bodies continue to offer incentives and expedited approvals for SCD therapies.
Increased Awareness & Screening Programs: Public health campaigns and neonatal screening initiatives are improving early diagnosis and timely intervention.
Market Segmentation:
By Disease Type:
Hemoglobin SS (HbSS)
Hemoglobin SC (HbSC)
Hemoglobin (HbS) Beta Thalassemia
Others.
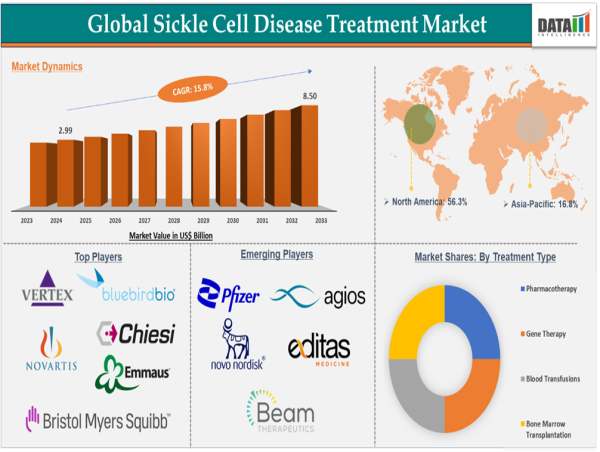
By Treatment Type:
Pharmacotherapy
Gene Therapy
Blood Transfusions
Bone Marrow Transplantation.
By Region:
North America
Latin America
Europe
Asia Pacific
Middle East
Africa.
Buy Now & Unlock 360° Market Intelligence:
Purchase Industry Subscription Today – Make Smarter Decisions Tomorrow:
Purchase Your Subscription to Power Your Strategy with Precision: https://www.datamintelligence.com/buy-now-page?report=sickle-cell-disease-treatment-market
Geographical Share:
North America dominates the market, driven by its robust healthcare infrastructure, the presence of major industry players, and substantial investments in research and development.
Africa and the Middle East represent high-potential markets given the high disease prevalence.
Asia-Pacific is steadily emerging, propelled by rising awareness and improved healthcare access.
Europe maintains a solid market share due to early diagnosis programs and gene therapy trials.
Key Market Players:
Key players leading innovation and competition in the SCD treatment market include:
Novartis AG
Vertex Pharmaceuticals Incorporated.
bluebird bio, Inc.
Emmaus Medical, Inc.
Bristol-Myers Squibb Company.
CHIESI FARMACEUTICI S.p.A.
Teva Pharmaceutical Industries Ltd.
Agios Pharmaceuticals, Inc.
Beam Therapeutics.
Editas Medicine
Novo Nordisk A/S
Pfizer Inc.
These companies are investing in new drug launches, clinical trials, and strategic collaborations to maintain competitive advantage and expand treatment portfolios.
Recent Developments:
United States
2025 – Vertex Pharmaceuticals, in collaboration with CRISPR Therapeutics, initiated Phase III trials for a one-time gene-editing therapy targeting SCD, aiming for FDA approval by late 2026.
2024 – Pfizer successfully completed the acquisition of Global Blood Therapeutics, enhancing its rare disease pipeline with access to the FDA-approved SCD treatment, Oxbryta®.
Japan
2025 – Otsuka Pharmaceutical announced a partnership with a global biotech firm to introduce a novel oral SCD therapy under clinical evaluation in Japan.
2024 – Japanese regulatory authorities approved the launch of a public SCD awareness and newborn screening program across major cities, strengthening early detection efforts.
Stay informed with the latest industry insights-start your subscription now: https://www.datamintelligence.com/reports-subscription
Conclusion:
The Global Sickle Cell Disease Treatment Market is on a transformative path, driven by innovations in precision medicine and supportive regulatory environments. As biopharma companies push boundaries in genetic therapies and governments amplify support through awareness and screening, the future of SCD treatment promises improved patient outcomes and enhanced quality of life worldwide.
Related Reports:
Wilsons Disease Treatment Market:
Liver Disease Treatment Market
Sai Kiran
DataM Intelligence 4Market Research
+1 877-441-4866
[email protected]
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
![]()