Highlights
- Imugene has received the US FDA clearance for the Investigational New Drug for a Phase 1 clinical study of its oncolytic virotherapy candidate.
- With this clearance, the company will begin enrolment of subjects as well as dose administration in the clinical study.
- The clinical study will involve patients with advanced or metastatic solid tumours.
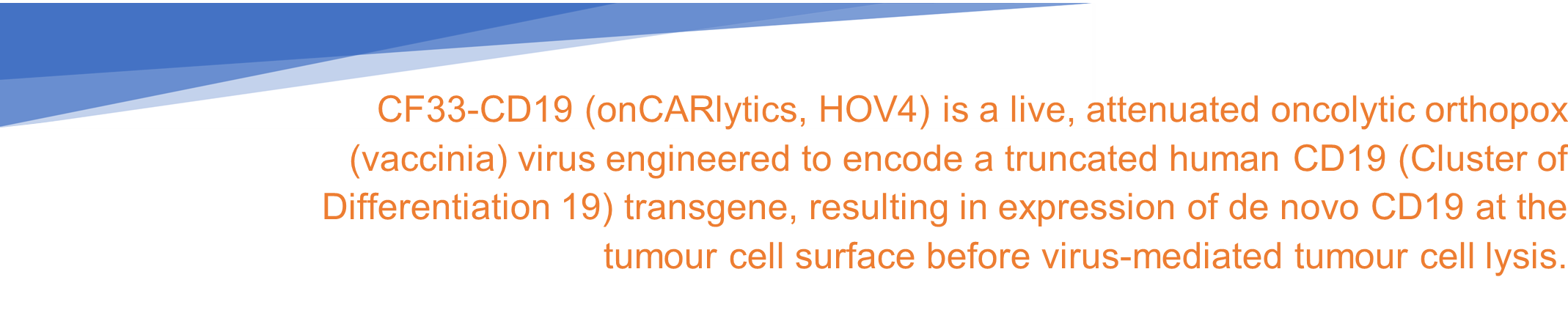
In the latest announcement, clinical-stage immunooncology company Imugene Limited (ASX: IMU), revealed that the United States Food and Drug Administration (FDA) has cleared its Investigational New Drug (IND) application for a Phase 1 clinical trial of its oncolytic virotherapy candidate, onCARlytics (on-CAR-19, CF33-CD19, HOV4).
With the IND clearance from the FDA, the ASX-listed firm can initiate enrolment of subjects as well as dose administration in the Phase 1 clinical study for the onCARlytics platform in patients having Advanced or Metastatic Solid Tumors.
The trial has been titled as “A Phase I, Dose Escalation and Dose Expansion, Safety and Tolerability Study of onCARlytics (CF33-CD19), Administered Intravenously or Intratumorally in Combination with Blinatumomab in Adults with Advanced or Metastatic Solid Tumors (OASIS).”
According to the company release, IMU’s CF33-CD19 oncolytic virus in combination with the CD19 targeting bispecific monoclonal antibody blinatumomab (Blincyto®) holds the potential to target and eliminate solid tumours which Blincyto® therapy cannot treat alone.
Remarks by IMU MD & CEO
 Data source: Company update
Data source: Company update
Understanding the onCARlytics study
Data source: Company update
Imugene is developing the virus for its use in combination with a CD19 targeting agent to treat advanced or metastatic solid tumours. According to the company, this trial will be a first-in-human (FIH) study in adult patients having advanced or metastatic solid tumours.
The study is being performed with the objective to analyse the safety and efficacy of two routes of administration of CF33-CD19, intratumoral (IT) injection and intravenous (IV) infusion, either alone or in combination with blinatumomab.
Blinatumomab is a bispecific CD19-directed CD3 T-cell engager [BiTE] specific to CD19 and CD3.
IMU stock performance
The company stock was trading at AU$0.127 on 18 May 2023, registering a 10.86% jump from the last close. IMU stock has gained more than 283% in past 5 years. The company has a market cap of AU$738.64 million.




