Summary
- Five companies have been selected under Operation Warp Speed (OWS) by the US administration to develop a COVID-19 vaccine, including AstraZeneca and Moderna.
- Under the OWS program, funding, assistance in the clinical trials, and support for manufacturing will be provided by the government to the selected vaccine programs.
- AstraZeneca and Emergent BioSolutions sign a USD 87 million deal for manufacturing AstraZeneca’s COVID-19 vaccine.
- Moderna advances late-stage development of the mRNA-1273 vaccine against COVID-19, to commence Phase 3 trial in July-2020.
Last month, the US proposed Operation Warp Speed (OWS) for massive COVID-19 vaccine testing attempt to meet year-end deadlines. The operation has the goal to have significant amounts (nearly 300 million doses) of an effective and safe vaccine to combat COVID-19 accessible to the US by early 2021.
Now, under the OWS program, five vaccine candidates have been chosen to provide funding. The vaccine developers under this program are AstraZeneca, Moderna, Johnson & Johnson, Merck, and Pfizer. The vaccine development programs selected under OWS will gain access to additional government funding, assistance in the clinical trial as well as manufacturing support.
The government funding is an essential keystone of drug development during the ongoing global health emergency, and without global access to a COVID-19 vaccine, it will be impossible to get the pandemic under control in the long-run.
As per the information provided by the Center for Infectious Disease Research and Policy (CIDRAP), the accelerated programs are funded through USD 10 billion from Congress and USD 3 billion directed for National Institutes of Health (NIH) research.
Let us now shed some light on the vaccine development of AstraZeneca and Moderna:
AstraZeneca and Emergent sign a deal for manufacturing COVID-19 vaccine
Cambridge, England-based healthcare player AstraZeneca Plc (NYSE:AZN) is a global biopharmaceutical company that focuses on the discovery, development and commercialisation of prescription medicines. AstraZeneca primarily focuses on the treatment of diseases in therapeutic areas of oncology, respiratory, cardiovascular, and renal & metabolism.
On 11 June 2020, AstraZeneca and Emergent signed an agreement, valued at nearly USD 87 million, to support the production of AZD1222, AstraZeneca’s vaccine to combat COVID-19. With this agreement, Emergent will offer contract development, analytical testing, drug substance process, technology transfer, performance qualification, and will also preserve specific large-scale production capacity across 2020 to support AstraZeneca’s COVID-19 vaccine candidate.
AZD1222, by AstraZeneca, is among the five vaccine contenders supported by the US government’s Operation Warp Speed to fasten the production and distribution of COVID-19 medical counter measures intending to have significant amounts of a safe and effective vaccine against novel coronavirus to be available by January 2021.
As part of Operation Warp Speed, Emergent will offer manufacturing services as well as capacity to pioneers of leading vaccine candidates nominated by the US government, for instance, AstraZeneca.

Moreover, the development services will be provided out of Gaithersburg product development facility of Emergent. However, large-scale production of drug substance will be done at the Baltimore Bayview facility that is planned for swift production of large quantities of vaccines as well as treatments during public health emergencies.
Stock Information:
The share price of AZN fell by 3.86% to close the day’s trade at USD 51.57 on 11 June 2020. The market capitalisation of AstraZeneca was approximately USD 135.35 billion.
Moderna Advances Late-Stage Development of mRNA-1273 Vaccine Against COVID-19
Cambridge-headquartered biotech player Moderna Inc (NASDAQ:MRNA) is pioneering mRNA therapeutics and vaccines to develop a new generation of transformative medicines for various disorders including some rare diseases, infectious diseases, heart disorders and cancers.
The Company has the potential to pursue a robust drug pipeline of novel drug candidates and has developed an mRNA vaccine that utilises the genetic material part of novel coronavirus (SARS-CoV-2) to stimulate an immune response from the human body.
HAVE YOU READ: Moderna Buzzing as it Enters Phase 2 Trial for COVID-19 vaccine
On 11 June 2020, Moderna disclosed the progress on the late-stage development of its mRNA vaccine candidate against COVID-19- mRNA-1273.
Moderna has finalised the Phase 3 clinical trial protocol based on the feedback received from the US FDA (Food and Drug Administration).
The clinical trial will be randomised, 1:1 placebo-controlled trial and is anticipated to include nearly 30,000 participants to be enrolled in the US. The Phase 3 clinical study is projected to be operated in collaboration with NIAID, a part of the NIH (National Institutes of Health).
The primary endpoint of this clinical trial will be the prevention of symptomatic COVID-19; key secondary endpoints include prevention of severe COVID-19 and prevention of infection by SARS-CoV-2.
Moderna disclosed that the primary efficacy analysis would be an event-driven analysis based on total participants with symptomatic COVID-19 infection. Based on the findings from the Phase 1 clinical trial, the 100 μg dose level was selected as the optimal dose level to increase the immune response while diminishing adverse reactions.

It is worth mentioning that Moderna has completed production of vaccine needed to commence the Phase 3 clinical trial and anticipates dosing in the study to start in July-2020. The Company disclosed that manufacturing of vaccine with Lonza had been scaled up and on track to provide 500 million - 1 billion 100 μg doses annually.
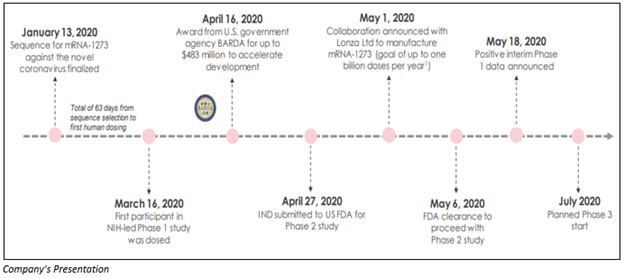
The below-represented figure shows how Moderna has progressed for the development of COVID-19 vaccine from January 2020 and how the Company succeeded in completing the clinical development as was planned initially.
ALSO READ: Prescription of Mental Health Medications Surges Amid COVID-19
Stock Information:
On 11 June 2020, MRNA stock settled at USD 60.20, climbing by 0.22% from its previous close. The market capitalisation of Moderna was approximately USD 22.35 billion.
Bottomline
The Operation Warp Speed (OWS) program launched by the US government to fasten the vaccine development will aid the selected healthcare companies to hit the ground running to find an effective COVID-19 vaccine. With the combine efforts, it can be anticipated that the healthcare companies will cut the mustard and soon come up with a vaccine to combat the deadly novel coronavirus.
HAVE YOU READ: Are We Very Near to the Breakthrough for Coronavirus Vaccine?