Summary
- The AstraZeneca licenced Jenner Institute of Oxford University developed vaccine candidate is in the most advanced stage of its clinical trials compared to its most other competitors being developed around the world.
- The vaccine’s latest development at the institute indicates that the rollout could be pushed to the end of the year.
- The early rollout of the vaccine during or before the onset of winter is important as there is a strong possibility of a resurgence of the pandemic during the winter season.
The Health Secretary of Her Majesty’s Government Matt Hancock has come up with a statement that the much talked about Oxford University Jenner institute COVID-19 vaccine ChAdOx1 nCovid-19 could be available by early next year. The vaccine which has been licensed to AstraZeneca is already being manufactured in large numbers so that as soon as the approval process is complete, it can be immediately be made available for vaccination. The country which has been witnessing a slow rate of economic recovery because of the continued safety precautions implementation could get a major boost if the vaccination process starts at the earliest. The coronavirus pandemic continues to spread across the United kingdom, despite the best efforts being made by the NHS, and till now has infected as many as 350,000 people out of which at least 41,500 people have already lost their lives.
The AstraZeneca and Oxford University deal for the vaccine
AstraZeneca got a major short in its arm when it was able to sign in a deal with the earliest developers of the coronavirus vaccine in the world. The University of Oxford's Jenner Institute has been working from as early as January of this year to develop a vaccine to counter the viral illness. The institute developed vaccine candidate which currently has the code name ChAdOx1 nCovid-19, showing encouraging results in its first trials on chimpanzees. The scientists at the institute were able to demonstrate that the non-human candidate when they were vaccinated with did not show their lungs getting damaged by this virus, nor was this virus able to multiply itself there. Taking this finding forward, the institute has now advanced it into the second stage clinical trials, and third stage clinical trials where it is applying it on thousands of humans volunteers from different races and age groups to test if this success can be replicated with the same level of efficacy. AstraZeneca and several of its partner manufacturers from across the world have already manufactured and stored billions of doses of this early candidate so that by the time the vaccine receives all its regulatory approvals there would be less lead time in government launching a vaccination programme for the general public. Previously both the company and Oxford University have been maintaining that the vaccine will be available by the month of September this year, but now it seems there could be a delay.
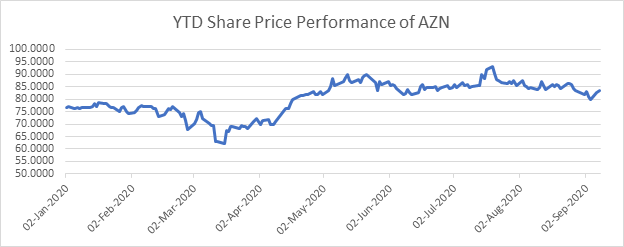
The performance of the shares of AstraZeneca on LSE since the beginning of the year
(Source – Thomson Reuters)
AstraZeneca Plc (LON:AZN) was hovering around GBX 8,344.00 on 8 September 2020 at 16:17 PM GMT+1, up by 0.83 per cent from its previous closing. The stock has given a YTD return of 8.09 per cent on the LSE.
Other major vaccine development candidates in the UK and around the world
In the UK, the government has entered into a supply agreement other than AstraZeneca for an alternative vaccine candidate. British pharma giant GSK and the French pharmaceutical giant Sanofi, also have a vaccine candidates in the development stage for this pandemic causing virus. For this candidate where the actual development is being done by Sanofi in association with GSK, making millions of doses of a powerful vaccine which both companies plan to roll out to the public in the early part of next year.
This vaccine candidate, a little different than the Oxford-AstraZeneca candidate is advanced in the sense that it is an Adjuvanted Vaccine candidate, meaning that there are inbuilt boosters in the vaccine that instigates the human immune system to produce more antibodies than it would otherwise do if only the vaccine is administered to a recipient. The antigen that has been used for the vaccine is based on recombinant DNA technology had the potential to prompt a superior human immune response compared to the Oxford-AstraZeneca vaccine or any other competitor candidates. Moreover, the distinctive aspect of this candidate is strongly placed amongst all other competing candidates, and it is receiving active support from Biomedical Advanced Research and Development Authority (BARDA), of the United States through funding and collaboration, signifying the trust the US has in this line of development.
Incidentally, Sanofi is also in the process of developing a medicine against COVID 19 along with US-based company Regeneron Pharmaceuticals which aims as repurposing rheumatoid arthritis drug Kevzara to treat patients who are severely ill because of the virus.
Other than these prominent two, there are also a number of vaccine candidates under development in various countries against COVID 19. Other than the Oxford- AstraZeneca and Sanofi- GSK candidates, some of the more important candidates are:
- The Moderna, mRNA-1273 candidate – Moderna, a United States-based drug development company, is working on an RNA based coronavirus vaccine as well. The company's candidate who entered into its third phase of clinical trials in July will see nearly 30,000 volunteers participating in the clinical trials process. The company, along with its partner company Catalent Inc plans to make 100 million doses of this candidate for the US market.
- CanSino Ad5-nCoV candidate – this candidate is being developed in China, by CanSino Biologics Inc in association with Beijing Institute of Biotechnology having satisfactorily evolved since development began. The vaccine is a Recombinant candidate and has already progressed through its phase- 1 trial and phase- 2 human trials with results showing an encouraging immune response against the COVID- 19 causing virus.
- Pfizer/BioNTech vaccine candidate – This vaccine is being developed under a joint venture agreement between American drug maker Pfizer and BioNTech, a German pharma company. It is an mRNA-based vaccine and is currently undergoing testing on volunteers in the United States and Germany, who are showing a strong immunological response against the virus.
- Inovio Pharmaceuticals/International Vaccine Institute candidate – Unlike above-mentioned candidates, this is an experimental DNA based vaccine which has already shown good results in early human stage trials. This candidate is currently undergoing Phase- 1 and Phase- 2 clinical trials in the US.





