@Kalkine Image
Summary
- North American stock markets rallied on Monday morning after Moderna claimed its Covid-19 vaccine is 94.5 per cent effective.
- Moderna claims that its vaccine candidate is stable at 2° to 8°Celcius (36° to 46° Fahrenheit) for 30 days.
- Moderna stock is up over 350 per cent this year.
- Precious metals gold and silver dropped as investors dumped safe assets and flocked to riskier stocks.
Canadian stock markets rallied on Monday (November 16) morning after biotech firm Moderna refueled Covid-19 vaccine hopes claiming 94.5 per cent efficacy in preventing the disease. Moderna stock (NASDAQ: MRNA, MRNA:US) was up ~16 per cent in pre-market trade on Monday. At the time of writing this, the stock was trading US$ 95.4, up 6 per cent.
The news comes a week after US pharmaceutical giant Pfizer (NYSE:PFE, PFE:US) and German biotech firm BioNTech (NASDAQ: BNTX, BNTX:US) announced 90 per cent efficacy in preventing Covid-19 during the phase 3 study trial for COVID-19 vaccine candidate.
As investors hailed Moderna vaccine news, the TSX Composite index went up by 77.11 points, or 0.46 per cent, to 16,752.75 in early Monday trade (9:33AM EST). The TSX Venture Composite index also gained 0.66 per cent to 741.21. The rise was also supported by jump in oil prices after data showed rebound in China and Japan, the world's second and third largest economies, respectively.
On Wall Street, the S&P 500 was up 0.77 per cent to 3,612.90 while the Nasdaq was up +0.50 per cent to 11,888.43. The Dow Jones was up 1.17 per cent to 29,824.67.
Covid-19 cases have soared past 54 million-mark, with over 1.3 million deaths, since the virus broke out in Wuhan, China, late last year.
Meanwhile, the World Health Organization director-general Tedros Adhanom Ghebreyesus said that the vaccine alone “will not end the pandemic,” but just complement the other coronavirus prevention tools.
Moderna COVID-19 Vaccine Claims
Reporting preliminary results from the COVE study with over 30,000 participants, Moderna claimed that its COVID-19 vaccine candidate mRNA-1273 is 94.5 per cent efficient.
Moderna is conducting the COVE study in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA). The study randomized 1:1 placebo-controlled trial of the mRNA-1273 vaccine (100 µg dose level) in participants aged 18 and above.
The biotech firm now intends to apply for an Emergency Use Authorization (EUA) with the US Food and Drug Administration (FDA) and other global regulatory agencies in the coming weeks. Moderna expects having the EUA informed by the final safety and efficacy data (with a median duration of at least two months).
Moderna further claimed that the vaccine remains stable at 2° to 8°Celcius (36° to 46° Fahrenheit) for 30 days and at room temperature for up to 12 hours. For long-term storage and shipping, the mRNA-1273 vaccine can be maintained at -20°C (-4°F) for up to six months.

Gold & Silver Slump
As investors dumped safe assets and flocked to riskier assets on the back of Moderna vaccine news, gold and silver rates fell in early trade on Monday.
Gold futures were down 0.14 per cent to US$ 1,886.40. Silver futures too dropped 0.56 per cent to US$ 24.637.
The TSX tech index doffed almost 0.8 per cent at the time of writing this, with Shopify (TSX:SHOP) down 2.67 per cent, the biggest price decline across the Toronto Stock Exchange.
Stock Watch: Moderna Inc. (NASDAQ: MRNA, MRNA:US)
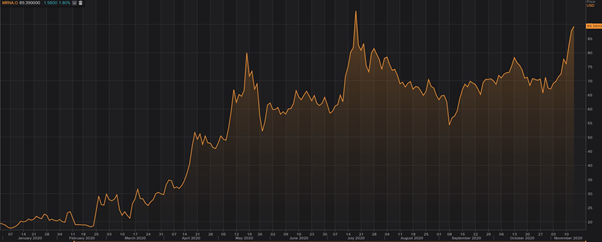
Moderna’s YTD price chart (as on November 16, 2020) / Source: Refinitiv, Thomson Reuters
Moderna stock is up over 350 per cent this year. Its current price to book ratio is 12.83 and price to cash flow is ratio is 49.3, as per data on the TMX portal. The company’s current market capitalization is above C$35 billion.
Its total revenue stood at US$157.9 million in third quarter 2020, up from US$17.0 million for the same quarter 2019. Net Loss stood at US$233.6 million in Q3 2020, as compared to US$123.2 million a year ago. The company ended the quarter with cash, cash equivalents and investments of US$3.97 billion.
On The Vaccine Front
Moderna is the second entity to post positive developments, a week after Pfizer and BioNTech announced its vaccine candidate for SARS-CoV-2, the virus that causes coronavirus, is 90 per cent effective in preventing COVID.
As per latest media reports, the United States alone could administer over 60 million doses by 2020-end post approval from authorities.
Meanwhile, the US Food and Drug Administration (FDA) approved emergency authorization for Eli Lilly's bamlanivimab, a Covid-19 monoclonal antibody treatment for adults with mild-to-moderate Covid-19 symptoms. The drug can be administered within 10 days of symptoms immediately after the patient tests positive for Covid-19, said the company.
A combined dose of GlaxoSmithKline’s GSK.L vaccine booster and Canadian drug producer Medicago’s experimental Covid-19 vaccine also reportedly produced virus-neutralizing antibodies.