Highlights
- Cynata is testing the safety and efficacy of CYP-006TK in diabetic foot ulcers (DFUs), also called diabetic wounds, in an ongoing clinical trial
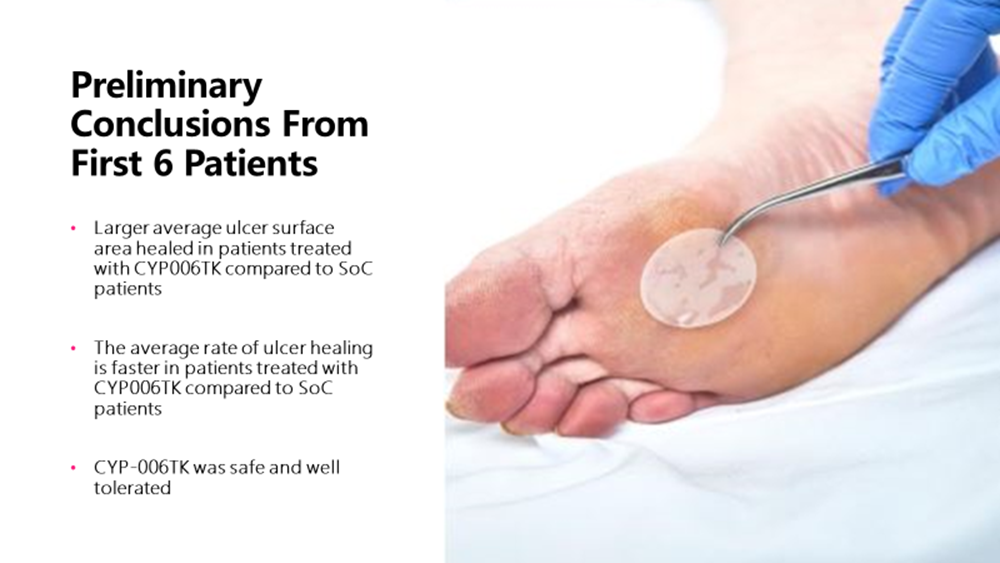
- Initial data from the first six patients is encouraging, demonstrating a notable decrease in average ulcer size in the three patients treated with CYP-006TK compared with the three patients receiving standard treatment
- The data also demonstrated that the average rate of ulcer healing was faster in patients treated with CYP-006TK compared with patients receiving standard treatment
Publicly listed Australian clinical stage stem cell company Cynata Therapeutics (ASX: CYP), which is developing therapies powered by the Company’s proprietary Cymerus™ therapeutic stem cell platform, is conducting clinical trials of its products in osteoarthritis (Phase 3) and diabetic foot ulcers (DFU). With respect to the latter, Cynata lately released a clinical trial update and reported positive initial data from the first six patients.
Besides the reduction in average ulcer size and faster average rate of ulcer healing in patients treated with CYP-006TK compared with those receiving standard treatment, CYP-006TK was also well tolerated and safe. This initial patient data is encouraging and points to safety and efficacy of CYP-006TK in chronic wounds, CYP reports. The clinical trial is continuing with planned enrolment of the full cohort of 30 patients by mid-2023. Let us take a quick look.
DFU Clinical trial review
The review is related to the first six patients with at least 28-day follow-up. Cynata has said that there has been a notable reduction in average ulcer size in the case of three patients that were treated using CYP-006TK as compared to patients that had been receiving standard care. The average rate of healing was also quicker in those being treated with CYP-006TK than those getting standard care. Notably, safety and tolerability aspects have been positive.
CYP-006TK, Cynata explains, leverages the proprietary wound dressing technology of TekCyte (licensed exclusively to Cynata Therapeutics) to topically deliver Cymerus mesenchymal stem cells (MSCs). This way MSCs are applied directly to the wound. CYP states that the patch technology simplifies topical delivery of MSCs.
Source: Company update
Progress so far
After successful completion of aspects like signing of a licence with TekCyte, receipt of ethics approval, and completing trial start up activities, Cynata is now into the recruitment and treatment phase of the trial designed to test the safety and efficacy of CYP-006TK in treatment of DFUs. The Company is aiming to recruit 30 patients and each patient's evaluation lasting for 24 weeks. Clinical trial sites were initially only in Adelaide but the Company recently announced expansion of the study to include sites also in Perth to increase recruitment rate and ensure the study is fully enrolled by around the middle of 2023.
The Company has said that the final data from the complete trial would be released around the end Q4 2023. It is pertinent to note that diabetes affects more than 400 million people globally. DFUs, also called diabetic wounds, occur in up to 34% of patients, causing more than 27,000 hospitalisations in Australia every year. The number of amputations and deaths due to diabetic foot disease is also high.
Cynata shares traded at a price of AU$0.155 at the time of writing on 8 May 2023, with market cap of over AU$26 million.