Summary
- Shares of Valneva jumped 16 per cent after the UK government procured 40 million additional doses of its Covid-19 vaccine.
- After the jump, the shares of Valneva SE hit a fresh all-time high on the Euronext Paris stock exchange.
Shares of France-based vaccine maker Valneva SE (EPA: VLA) jumped over 16 per cent on Monday, 1 February, after the UK government said it procured 40 million additional doses of their Covid-19 vaccine. The stock of Valneva extended the gains after opening 10 per cent higher in the morning trade.
With the deal between the UK government and Valneva, it is scheduled that the country would receive 60 million doses of Valneva Covid-19 vaccine in the H2 2021 and the rest 40 million in 2022. All the vaccine shots are set to be manufactured at the Livingston, Scotland facility of Valneva.
Following the agreement, the UK has now secured early access to more than 400 million vaccine shots that are likely to be received in 2021 and 2022.
Valneva shares (1 February)
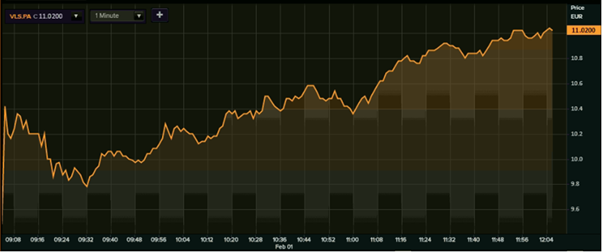
(Source: Refinitiv, Thomson Reuters)
Stock soars 16%
After the development, shares of Euronext Paris-listed component of Valneva SE rocketed more than 16 per cent from the previous close. According to the data available with the exchange, the stocks of Valneva touched a fresh all-time high of EUR 11.06 per share after rising as much as 16.67 per cent from the previous close of EUR 9.48 apiece.
With today’s appreciation in the share prices, the stock of Valneva stands with a cumulative monthly gain of nearly 48 per cent. Over the course of last year, Valneva shares have already recognised a gain of around 200 per cent. In fact, after today’s jump, the shares of Valneva SE hit a fresh all-time high on the Euronext Paris stock exchange.
Strengthening vaccine portfolio
For the next two years, the UK vaccine portfolio has access to 407 million doses of Covid-19 vaccines produced by different pharmaceutical companies, including Valneva, AstraZeneca/Oxford University, Pfizer/BioNTech, GSK/Sanofi Pasteur, and Moderna.
The additional doses are likely to supplement the vaccine availability and may provide flexibility if there is a requirement to revaccinate any individual. The Livingston manufacturing facility of Valneva has recently begun the large-scale production of Covid vaccines. Last year, the UK government had made a multi-million investment in the Valneva’s manufacturing unit.
The vaccine being developed by Valneva is presently undergoing analysis in the Phase 1 & 2 clinical trials. The Valneva vaccine will get an approval from the UK Medicines and Healthcare products Regulatory Agency (MHRA) only after ticking the desired level of efficacy, necessary safety, and minimised level of adverse effects.
With the production capacity of up to 250 million doses per year, the vaccines being manufactured at the Livingston site of Valneva can be distributed quickly upon approval from the drug regulator.
The deal is another weapon in the national arsenal to fight the coronavirus pandemic ensuring sufficient supplies for 2021 and thereafter, said Business Secretary Kwasi Kwarteng.
The Valneva vaccine will be rolled out across the four nations as quickly as possible, if it receives clinical consent and authorisation from the UK health regulator, said Health Secretary Matt Hancock.