Summary
- Pharma major AstraZeneca received the approval of Chinese regulators for its small cell lung cancer drug Imfinzi
- Regulators approved the drug after its phase 3 CASPIAN trials showed clinically meaningful improvement to patients’ survival rates.
- About 15 per cent of lung cancer is classified as small cell lung cancer
AstraZeneca (LON: AZN), the pharmaceutical major, which has been news for last some time due to its Covid-19 vaccine, is once again creating buzz after receiving approval in China for its small cell lung cancer drug Imfinzi. Shares of the company rebounded in early trade this morning after falling on 19 July despite the news.
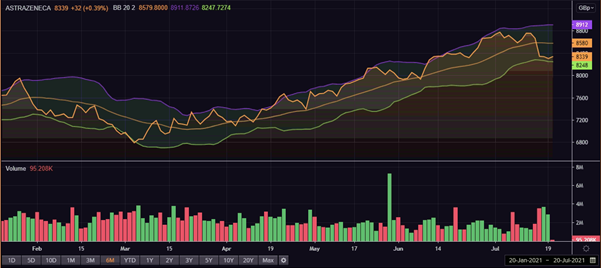
AstraZeneca (LON:AZN) share price performance
AstraZeneca’s shares were trading at GBX 8,340.00, up by 0.40 per cent on 20 July at 08:05 AM GMT+1, rebounding after a pullback on 19 July amid broader negative market sentiment despite the news.
Meanwhile, the FTSE 100 index, which it is a part of, was trading at 6,898.38, up by 0.79 per cent.
AstraZeneca’s shares had ended at GBX 8,307.00, down by 0.40 per cent on 19 July. The shares fell on Monday over concerns about rising covid-19 cases, thereby dragging the FTSE 100 index and pharma companies in the sector in the red.
(Image Source: Refinitiv)
AZN’s market capitalisation stood at £109.053 billion, and its year to date return is 14.20 per cent.
Also Read: Astrazeneca's Tagrisso Drug Gets Nod for Lung Cancer Treatment
AstraZeneca’s Imfinzi drug approval in China
The pharma company’s drug Imfinzi (durvalumab) received approval from the Chinese regulator National Medical Products Administration, as the first line of treatment for small cell lung cancer.
Imfinzi will be applied on adult patients who have been diagnosed with extensive stages of the disease. The treatment is recommended to be made in conjunction with standard of care platinum chemotherapy.
Positive results stemmed from the company’s CASPIAN phase 3 trials formed the basis for the consent from the regulator, which showed a statistically significant and clinically meaningful improvement in total endurance rates when a blend of the drug and chemotherapy were offered as a treatment as an alternative of only chemotherapy process.
Small cell lung cancer
About 15 per cent of lung cancer is classified as small cell lung cancer (SCLC), moreover, approximately 67 per cent of SCLC diagnosed patient are diagnosed with the extensive stage of the disease. SCLC is one of the most aggressive forms of lung cancer and also a fast-growing type of lung cancer.
Only 3 per cent of patients diagnosed with the extensive stage of SCLC survive after five years post diagnosis. Meanwhile, the survival rate is 7 per cent for SCLC patients.
Also Read: EU approves AstraZeneca Drug Koselugo for Treating NF1 in children
.jpg)




