GA, UNITED STATES, February 5, 2025 /EINPresswire.com/ -- The temporomandibular joint (TMJ) develops from neural crest cells which originate from the neuroectoderm. Based on the scientific hypothesis that the TMJ, as a "neurogenic joint," is more susceptible to peripheral nerve regulation and innervation.
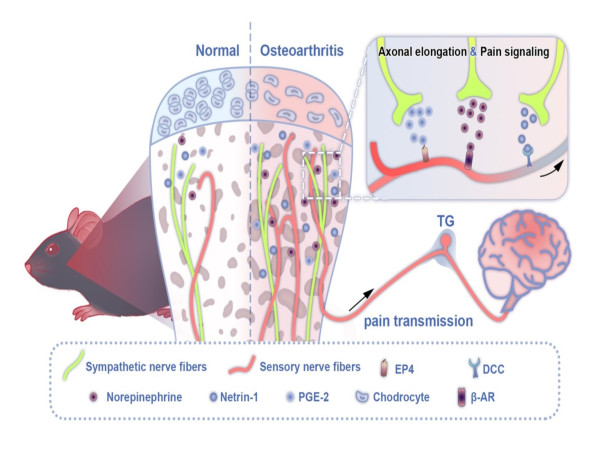
The temporomandibular joint (TMJ) develops from neural crest cells which originate from the neuroectoderm. Based on the scientific hypothesis that the TMJ, as a "neurogenic joint," is more susceptible to peripheral nerve regulation and innervation. Professor Kai Jiao's team at Tangdu Hospital of Airforce Medical University has previously confirmed the innervation and functional modulation of the condylar cartilage and subchondral bone of the TMJ by peripheral nerves1-8. Their latest study (DOI: 10.1038/s41368-024-00336-6), published on January 7, 2025, in the International Journal of Oral Science, reveals the pivotal role of sympathetic nerves in modulating temporomandibular joint osteoarthritis (TMJ-OA) pain and highlights a previously unexplored interaction between the sympathetic and the sensory nervous system, providing a fresh perspective on pain regulation in this condition. This study provides new evidence for the TMJ as a "neurogenic joint" and, for the first time internationally, elucidates the mechanism and intervention targets of sympathetic-sensory interaction in promoting TMJ-OA pain through bone-sensing mechanisms. It offers new support for the hypothesis that the TMJ is a "neurogenic joint" and provides novel therapeutic targets for the treatment of TMJ-OA pain.
TMJ-OA is a severe form of temporomandibular disorders (TMDs), affecting over 40 million patients in China. The most common and debilitating symptom reported by TMJ-OA patients is orofacial pain. This pain significantly impairs their ability to chew, speak, express emotions, swallow, and perform other oral functions, leading to considerable physical discomfort and psychological distress. However, there is still lack of effective clinical strategies for precise pain relief in TMJ-OA patients, largely due to an inadequate understanding of the mechanisms underlying TMJ-OA pain.
Notably, unlike other joints in the body that develop from the mesoderm, the TMJ in the orofacial region originates from neural crest cells of the neuroectoderm. Given its shared origin with the nervous system, can the TMJ be considered a "neurogenic joint" that is more susceptible to regulation and innervation by peripheral nerves? Previous studies by Professor Kai Jiao's team have already confirmed the innervation and functional modulation of the condylar cartilage and subchondral bone of the TMJ by peripheral nerves1-8. In this study, based on the hypothesis that the TMJ, as a "neurogenic joint," is more prone to peripheral nerve innervation, Jiao's team innovatively constructed mouse models of TMJ-OA pain with peripheral sympathetic nerve activation and blockade. The results showed that during the development of TMJ-OA, the innervation of sympathetic nerves in the subchondral bone of the condyle increases prior to the increase in sensory nerves. The increased sympathetic nerves promote the growth and activation of sensory nerves in the subchondral bone of the condyle by releasing norepinephrine (NE), thereby contributing to the onset of TMJ-OA pain. This process can occur independently or synergistically with neuroactive factors such as netrin-1 and prostaglandin E2 (PGE2). Further in-depth research has revealed the molecular mechanisms through which sympathetic nerve signals promote the ingrowth and activation of sensory nerve endings, clarifying the intervention targets for alleviating TMJ-OA pain by blocking sympathetic signals.
Prof. Kai Jiao, the corresponding author, emphasized the transformative potential of the findings: " Our study is the first internationally to uncover a novel mechanism through which the interaction between sympathetic and sensory nerves promotes TMJ-OA pain via bone-sensing effects. This discovery provides precise targets for clinical interventions aimed at treating TMJ-OA pain. Furthermore, our research deepens the understanding of how peripheral nerves innervate and modulate the TMJ condyle, offering new evidence to support the hypothesis that the TMJ is a "neurogenic joint." It also opens up fresh avenues for investigating the innervation and regulation of the TMJ."
The discovery of the sympathetic-sensory nerve crosstalk in TMJ-OA pain offers new possibilities for clinical practice. Targeting this interaction could lead to more effective pain management strategies, potentially improving outcomes for millions of patients worldwide. As research continues, future efforts will likely focus on developing therapies that can disrupt this crosstalk, which could revolutionize the management of TMJ-OA and related disorders.
References
1. Jiao, K. et al. Norepinephrine Regulates Condylar Bone Loss via Comorbid Factors. Journal of dental research 94, 813-820, doi:10.1177/0022034515577677 (2015).
2. Jiao, K. et al. β2-Adrenergic signal transduction plays a detrimental role in subchondral bone loss of temporomandibular joint in osteoarthritis. Scientific reports 5, 12593, doi:10.1038/srep12593 (2015).
3. Jiao, K. et al. Activation of α2A-adrenergic signal transduction in chondrocytes promotes degenerative remodelling of temporomandibular joint. Scientific reports 6, 30085, doi:10.1038/srep30085 (2016).
4. Sun, JL, Jiao K*. et al. Conditional deletion of Adrb2 in mesenchymal stem cells attenuates osteoarthritis-like defects in temporomandibular joint. Bone 133, 115229, doi:10.1016/j.bone.2020.115229 (2020).
5. Sun, JL, Jiao K*. et al. MicroRNA-29b Promotes Subchondral Bone Loss in TMJ Osteoarthritis. Journal of dental research 99, 1469-1477, doi:10.1177/0022034520937617 (2020).
6. Wan, QQ, Jiao, K*. et al. Crosstalk between Bone and Nerves within Bone. Advanced science 8, 2003390, doi:10.1002/advs.202003390 (2021).
7. Wan, QQ, Jiao, K*. et al. Simultaneous Regeneration of Bone and Nerves Through Materials and Architectural Design: Are We There Yet?.Advanced Functional Materials 30.1-33 doi:10.1002/adfm.202003542 (2020).
8. He JY, Jiao K*. et al. Upregulated mitochondrial dynamics is responsible for the pro-catabolic changes of chondrocyte induced by α2-adrenergic signal activation. Cartilage 15, 440-452 doi:10.1177/19476035231189841 (2023).
DOI
10.1038/s41368-024-00336-6
Original URL
https://doi.org/10.1038/s41368-024-00336-6
Founding information
National Nature Science Foundation of China 82471000 (to K.J.). National Key Research and Development Program 2023YFC2509100 (to K.J.). National Nature Science Foundation of China 82170978 (to K.J.). National Nature Science Foundation of China 82325012 (to L.N.N.).
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
![]()