Highlights
- Ten patients were enrolled in the Phase 1b trial of azer-cel, divided into two cohorts: Cohort A (azer-cel and lymphodepletion) and Cohort B (azer-cel, lymphodepletion, and IL-2).
- Three patients (two from Cohort B, one from Cohort A) achieved complete responses (CRs).
- CRs in Cohort B have been sustained for over 120 days in one patient and over 90 days in another.
- The trial is ongoing, with all four patients in Cohort B still participating.
- To date, treatment with azer-cel has been safe and tolerable.
Imugene Limited (ASX:IMU) has released encouraging results from the Phase 1b clinical trial with azer-cel, an allogeneic off-the-shelf CD19 CAR T. To date, 10 patients with relapsed/refractory diffuse large B cell lymphoma (DLBCL) have been treated with azer-cel under the trial. In the Cohort A, six patients were treated with lymphodepletion and azer-cel, while in the cohort B, the remaining four patients were treated with lymphodepletion, azer-cel and interleukin 2 (IL – 2).
The first two patients in cohort B and one patient in cohort A reported a CR. The CRs in Cohort B have been sustained for over 120 days in one patient and over 90 days in another, suggesting the durability of the response.
All 4 patients in Cohort B are currently ongoing in the trial with 1 patient still awaiting evaluation, additional patients to be enrolled into this cohort.

More on the results
In the Phase 1b trial, patients are being enrolled at 15 cancer centres in the US, with plans to expand to up to five sites in Australia. All participants had previously failed multiple treatments, including autologous CAR T therapies.
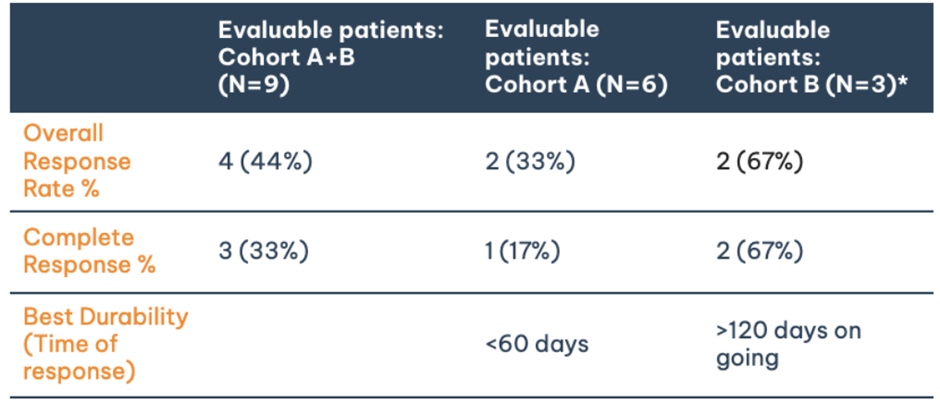
Image source: Company update
From Cohorts A and B, nine patients have qualified for the minimum 28-day scan, while one patient from Cohort B has completed treatment and is waiting for the 28-day scan.
The company plans to continue enrolling additional patients in Cohort B and will monitor their responses to assess durability. The goal is to compile a comprehensive package for submission to the FDA in preparation for a potential Phase 2/3 registrational trial.
If successful, azer-cel could become the first approved allogeneic CAR T cell therapy for blood cancers, highlights the company update.
The company also plans to explore expanding the research to treat solid tumours by combining azer-cel with the onCARlytics program. This expansion could unlock a significant market for azer-cel, addressing 90% of cancers beyond blood cancers.
About the trial
The Phase 1b azer-cel trial is an ongoing, open-label, multi-centre study conducted in the US and Australia. It targets patients with DLBCL, a fast growing and aggressive form of non-Hodgkin’s lymphoma (NHL), with nearly 80,500 new cases annually.
Patients treated with azer-cel and lymphodepletion (LD) have shown an acceptable safety profile. Additionally, patients in Cohort B, who received azer-cel, IL-2 and LD, have demonstrated sustained responses and clinically meaningful activity.
IMU shares soar
IMU shares jumped nearly 30% to AU$0.080 apiece at the time of writing on 2 September 2024.





