Summary
- Backed by an international leadership team with extensive commercialisation expertise in the sector, Imugene has developed a robust product portfolio, comprising four potential drugs.
- In 2020, Imugene intends to progress with Phase 1 trials of CF33 & PD1-Vaxx and continue enrolling into the open-label Phase 2 trial of HER-Vaxx in the fight against gastric cancer.
- IMU stock has delivered a robust return of over 60 per cent over the last three months (as on 7th July 2020).
Australian biotech player, Imugene Limited (ASX:IMU) deserves closer attention for marking remarkable progress towards the development of its exclusive product pipeline.
Backed by an international leadership team with extensive commercialisation expertise in the sector, Imugene has developed a robust product portfolio, comprising four potential drugs - VAXinia (CF33), CHECKvacc (CF33 & aPDL1), HER-Vaxx (HER-2) and PD1-Vaxx (PD-1). While HER-Vaxx and PD1-Vaxx are the Company’s B-cell cancer immunotherapies, VAXinia and CHECKvacc are oncolytic viruses directed towards a variety of solid cancers.
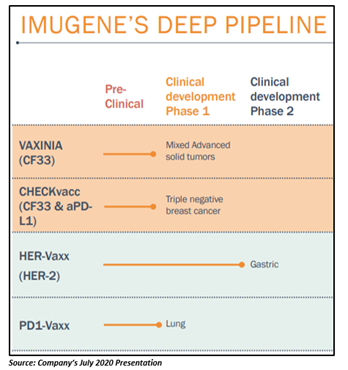
This year, Imugene intends to progress with Phase 1 trials of CF33 & PD1-Vaxx and continue enrolling into the open-label Phase 2 trial of HER-Vaxx in the fight against gastric cancer.
CF33’s Constructs - VAXinia and CHECKvacc
Imugene’s chimeric vaccinia (pox) virus, CF33 has been developed by Professor Yuman Fong at Los Angeles-based City of Hope Comprehensive Cancer Center. CF33 includes two additional genes inserted into the virus, hNIS (Human Sodium-Iodide Symporter) gene to facilitate imaging to chase the virus in vivo and mediate targeted radiotherapy, as well as anti PD-L1 to allow enhancement of anti-cancer immunotherapy.
Imugene has both CF33 candidates for development – the armed construct which combines CF33 with PD-L1 inhibition and hNIS (CHECKvacc) and the unarmed construct (VAXinia).
Both CHECKvacc and VAXinia constructs have been shown in pre-clinical studies to shrink injected and distant tumors, not directly targeted but in the metastatic spread. Besides, pre-clinical data has demonstrated that CF33 outperforms Amgen or Genelux oncolytic viruses in cancer growth inhibition.
Imugene is planning to undertake a VAXinia Phase 1 MAST (Mixed Advanced Solid Tumors) study in patients suffering from metastatic solid tumors or selected advanced tumors to evaluate the tolerance and safety of CF33-hNIS, administered intravenously (IV) or intratumorally (IT).
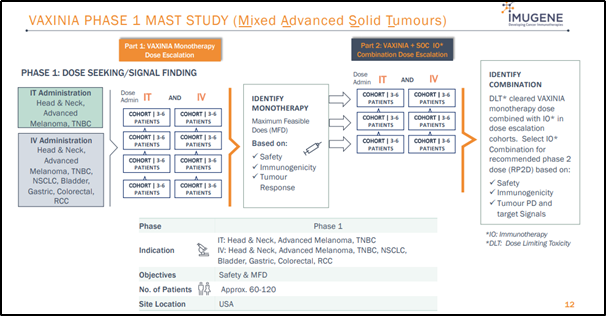
Source: Company’s July 2020 Presentation
Moreover, Imugene has also planned CHECKvacc Phase 1 trial in Triple Negative Breast Cancer (TNBC) patients. It is worth noting that pre-clinical studies undertaken at City of Hope have shown encouraging results in TNBC patients when an immune checkpoint inhibitor (especially a PD-L1 inhibitor) is combined with CF33 to generate CHECKvacc.
PD1-Vaxx
The PD1-Vaxx is Imugene’s second B-cell immunotherapy, which has been designed to treat tumors like lung cancer by interfering with PD1/PD-L1 binding and interaction, and deliver an anti-cancer effect akin to Keytruda, Opdivo and the other immune checkpoint inhibitor monoclonal antibodies.
PD1-Vaxx has shown encouraging responses and efficacy in preclinical studies, with strong inhibition of tumor growth in mouse models of colorectal cancer.
Imugene has planned to carry out Phase 1 clinical trial of PD1-Vaxx in CY2020, targeting NSCLC (non-small cell lung cancer), the most common type of lung cancer accounting for ~80% of cases. The Phase 1 trial involves testing of three different doses to identify safety, immunological data, and preliminary investigation of an optimal Phase 2 dose for the expansion stage of the study. Full study details can also be found on clinical trials.gov under study ID: NCT04432207
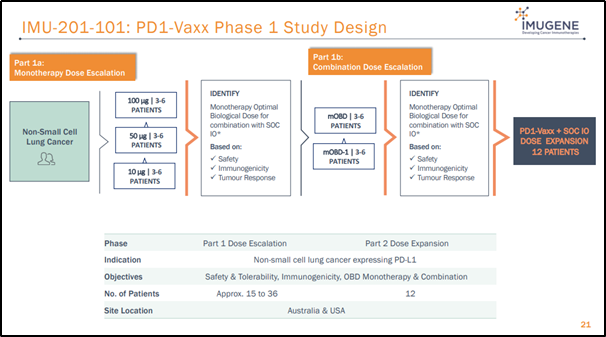
Source: Company’s July 2020 Presentation
Must Read! Imugene’s Preclinical New Mimotope Cancer Vaccine Research Published in Prestigious Journal
HER-Vaxx
HER-Vaxx is Imugene’s most advanced B-cell immunotherapy, which is under development for the treatment of HER2+ gastric cancer and holds the potential to treat other HER2-overexpressing cancers. In pre-clinical studies as well as the completed Phase 1b study, HER-Vaxx has been shown to stimulate a potent polyclonal antibody response to HER2/neu receptor.
Imugene’s HER-Vaxx Phase 1b trial returned robust results with no toxicity or safety issues, with all patients demonstrating increased antibody responses. The Company is now progressing with the recruitment of patients in Phase 2 trial being conducted at various sites across Eastern Europe and India.
Imugene has already received a go-ahead from Independent Data Monitoring Committee (IDMC) for the Phase 2 trial, with committee confirming no safety concerns from HER-Vaxx.
Way Ahead
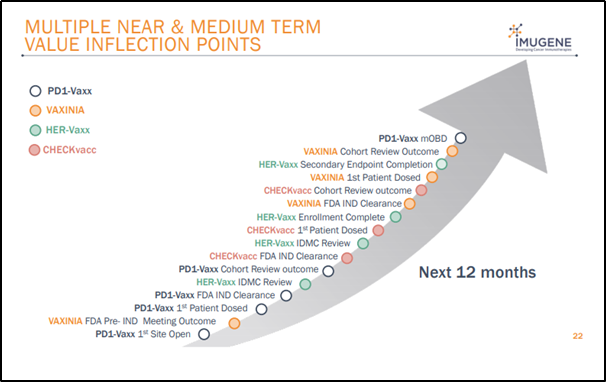
Source: Company’s July 2020 Presentation
IMU traded at $0.035 on 8 July 2020 (11:56 AM AEST).




