Cellular Therapy Emerges as a Front-Runner in Medical Innovation
TAOYUAN, Oct. 21, 2024 /PRNewswire/ -- Cellular therapy, a revolutionary approach where cells are manipulated outside the body and then reintroduced to treat or prevent diseases, has been recognized in recent years as a highly promising treatment in the medical field. Chang Gung Memorial Hospital in Taiwan has been at the forefront of this innovation since 2018, when it established a Good Tissue Practice (GTP) laboratory with six cell processing labs adhering to international PICS/GMP cleanliness standards. The hospital actively supports clinical trials for various immune cells and regenerative medicine, with its Department of Laboratory Medicine certified by the College of American Pathologists (CAP) and responsible for issuing quality control reports for cell production processes.
Chang Gung Memorial Hospital utilizes synthetic cancer cell antigens to train patients' immune cells to recognize and fight cancer cells. After multiplying, these immune cells are reintroduced into the body to eliminate cancer cells, all while using chemotherapy and radiotherapy to improve treatment outcomes. (Photo courtesy of Chang Gung Memorial Hospital)
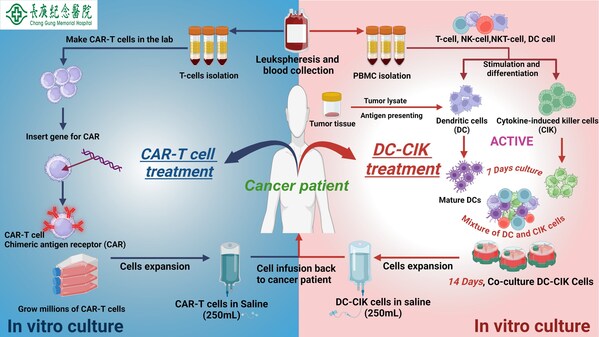
Autologous Dendritic Cell-Cytokine Induced Killer Cell Immunotherapy (DC-CIK)
Dr. Wei-Chen Lee, Vice-Superintendent at Chang Gung Memorial Hospital, Linkou, a leading institution in liver transplantation in Taiwan, has been researching autologous dendritic cells (DC) for liver cancer treatment since 1998. His therapy has achieved remarkable results, with no tumor growth post-treatment and a disease control rate of 70-80%. His team is now advancing research on cancer vaccines, aiming to initiate treatment immediately post-surgery to prevent cancer recurrence.
Professor John Yu from the Institute of Stem Cell and Translational Cancer Research (ISCTCR) has developed a novel three-stage "apexNK" expansion technology for natural killer (NK) cells. Combined with a high-efficiency viral transduction method, this technology produces new CAR-NK cells, overcoming the limitations of traditional CAR therapies in treating solid tumors. Director Shuen-Iu Hung from the Cancer Vaccine and Immune Cell Therapy Core Laboratory has completed Phase I clinical trials targeting tumor neoantigens for solid cancer treatment, paving the way for future applications in metastatic and multidrug-resistant tumors.
CAR-T Cell Therapy: A Living Drug Revolutionizing Cancer Treatment
CAR-T cell therapy, or chimeric antigen receptor T-cell therapy, represents a groundbreaking immunotherapy that combines gene therapy and cellular therapy. Dubbed the "living drug," the treatment modifies the patient's immune T-cells genetically, enabling them to precisely target and eliminate specific cancer cells. These engineered and expanded T-cells are then reintroduced into the patient's body. The therapy has demonstrated remarkable success in treating acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL), earning accelerated approval from the U.S. Food and Drug Administration (FDA).
Huahua, a 13-year-old, and Baobao, an 8-year-old, both boys, serve as examples. Both were diagnosed with ALL and later developed extramedullary relapse, with Huahua experiencing bilateral testicular enlargement and Baobao showing enlargement of the right testicle, accompanied by an increase in minimal residual disease (MRD).
Faced with these challenging cases, Dr. Shih-Hsiang Chen, Deputy Director of the hospital's Pediatric Department, noted the limited effectiveness of traditional hematopoietic stem cell transplantation for extramedullary relapse. Local treatments such as orchidectomy or radiotherapy could also significantly impact the reproductive function of pediatric patients. After thorough discussions with the patients' families, the decision was made to proceed with CAR-T cell therapy. Following treatment, both patients experienced only mild fever and fatigue and were discharged after approximately two weeks. They continue to receive outpatient follow-up care.
Dr. Cheng-Hsun Chiu, also a Vice-Superintendent at the hospital, said that advancements in cancer treatment have transformed the landscape, evolving from traditional radiotherapy and chemotherapy to targeted therapy, bone marrow transplantation, and now, pioneering cellular therapies like DC-CIK and CAR-T. Looking ahead, the evolution of immune cell therapy promises to usher in more technological breakthroughs, offering patients a broader array of treatment choices and significantly enhancing both treatment efficacy and quality of life.





