Foldax® Inc., a leader in heart valve innovation, today announced that the Indian Central Drugs Standard Control Organization (CDSCO) approved its TRIA™ Mitral Valve. Dolphin Life Science India LLP will locally manufacture the TRIA Mitral Valve in India.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250605200811/en/
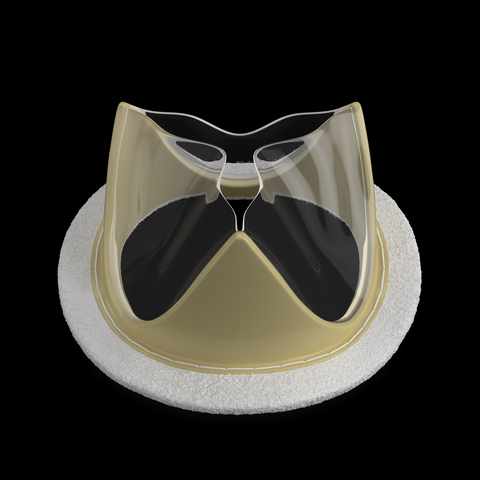
The TRIA Mitral Valve is made from proprietary LifePolymer material, developed specifically for heart valve use and engineered at a molecular level to be calcium-resistant, biostable and biocompatible. The valves are robotically manufactured for precision and consistency at scale.
Traditional valves made from animal tissue are prone to calcification and degradation, limiting their durability and often leading to repeat surgeries, especially for younger patients. Mechanical valves, while durable, are prone to thrombosis and require lifelong anticoagulation therapy that can impact a patient’s long-term quality of life. Foldax’s vision for its novel polymer heart valves is to address the limitations of tissue and mechanical valves by making them durable, with the goal of avoiding the requirement for lifelong anticoagulation.
The company’s portfolio of valves in development (transcatheter and surgical), including robotic implantation capabilities, is based on its proprietary LifePolymer™, a polymer material developed specifically for heart valve use and engineered at a molecular level to be calcium-resistant, biostable, and biocompatible. All Foldax valves are robotically manufactured for precision and consistency at scale. Clinical research on the TRIA Mitral Valve demonstrates favorable safety, stable hemodynamics, and meaningful improvements in patient quality of life.1
“My experience with the TRIA Mitral Valve has shown me that there is now a compelling alternative for a diverse patient population, including those as young as 18 years old and women of childbearing age, many of whom suffer from mitral valve damage caused by rheumatic fever,” said Kaushal Pandey, MD, Principal Investigator of the TRIA Mitral Valve India clinical trial and Cardiac Surgeon at P.D. Hinduja Hospital in Mumbai.
“Gaining approval with our partner Dolphin Life Sciences for the world’s first polymer heart valve marks a defining moment in the evolution of heart valve therapy and disrupts long-standing paradigms in the heart valve industry,” said Ken Charhut, Executive Chairman and CEO of Foldax. “We intend to extend our unique platform utilizing LifePolymer material and proprietary robotic manufacturing into additional applications in transcatheter devices and other valve positions. We look forward to making a transformative impact in India and other geographies where we can fill critical unmet clinical needs.”
*The TRIA Mitral Valve is for investigational use only and is not available for commercial sale in the U.S.
About Foldax
Headquartered in Salt Lake City, Utah, Foldax is reinventing every aspect of the heart valve – from material to design to manufacturing – to develop surgical and transcatheter valves with the potential to last a lifetime, addressing limitations of tissue and mechanical valves.
Foldax investors include Angel Physicians Fund, Biostar Capital, Caltech, Glenview Capital, Kairos Ventures, Memorial Care Innovation Fund and Sayan Bioventures. For more information on the TRIA Mitral Valve and Foldax’s commitment to revolutionizing heart valve care, visit www.Foldax.com.
References:
- Isaac George, MD. Surgical Mitral Valve Replacement with a Novel Polymeric Valve: 30-Day Results from an Indian Clinical Study of the Foldax TRIA Valve. Presented at New York Valves: The Structural Summit, June 5-7, 2024, New York, New York.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250605200811/en/
![]()



