Highlights
- Radiopharm has joined hands with Lantheus to develop a nanobody.
- The company is set to start a Phase 1 therapeutic trial in patients with NSCLC.
- Radiopharm gained the imaging rights of the nanobody from NanoMab for China.
Radiopharm Theranostics (ASX:RAD) has entered two strategic agreements with Lantheus and NanoMab, respectively.
The collaboration agreement with Lantheus is for the mutually beneficial development of nanobody NM-01. Under the agreement with NanoMab, the company gained the imaging rights of NM-01 in China and global IP rights.
Agreement with Lantheus to develop NM-01
Radiopharm and Lantheus have entered an agreement, under which the latter will deliver the diagnostic product candidate of NM-01 for use in the former’s therapeutic clinical studies. NM-01 will be used to evaluate PD-L1 expression during patient selection.
The agreement, which is effective immediately for a seven-year period, is likely to be funded from existing cash reserves.
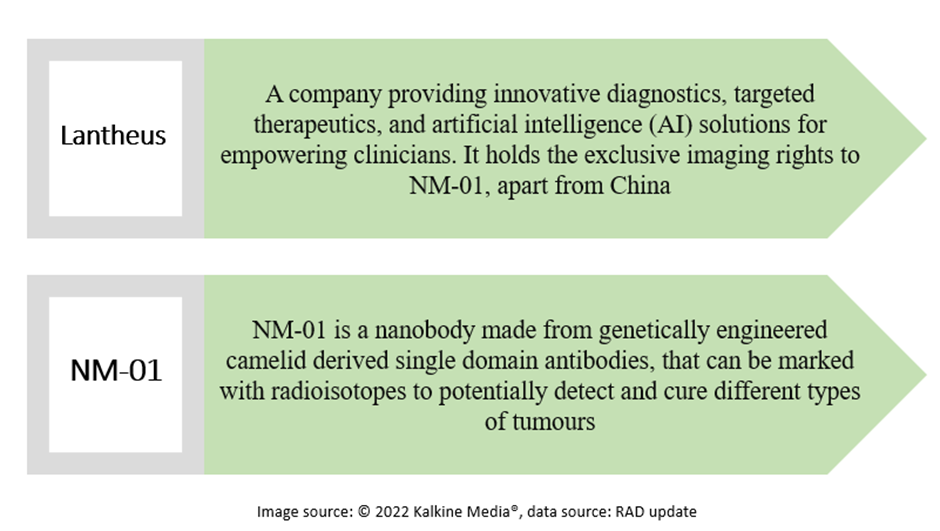
Lantheus has been studying PD-L1 expression in non-small cell lung cancer (NSCLC) patients under a recently started Phase 2 clinical trial of NM-01.
Radiopharm plans to study patients with PD-L1 + NSCLC under a Phase 1 therapeutic study in Australia.
The partners have reached an agreement to cross-reference each other's data to expedite the development plans for the PD-L1 assets. This includes the development and regulatory process with major governing agencies including the US FDA.
Further, according to the terms of the agreement, the partners also have the choice to cover additional assets and prospective licensing opportunities in Radiopharm's pipeline as part of an extended partnership.
Agreement with NanoMab for NM-01 imaging rights
Radiopharm has gained the imaging rights of NM-01 from NanoMab for the strategic Chinese market and global IP rights for any therapeutic use (earlier a licensing right).
As part of a broader partnership with NanoMab Ltd, Radiopharm's acquisition of the NanoMab PDL1 IP will be cost-free.
Commenting on the collaboration, Lantheus' Chief Business Officer Etienne Montagut said, "We believe NM-01's unique potential to evaluate patients before, during, or after treatment with checkpoint inhibitors will assist Radiopharm in the optimisation of the development of its immuno-oncology therapy."

Radiopharm has a market capitalisation of AU$53.64 million, and its shares traded at AU$0.21 on 8 August 2022.



