Highlights
- CHM received positive feedback for CHM 2101, a 3rd generation autologous CAR T cell therapy, from the US FDA
- The first patient was dosed with the CHM 0201 (CORE NK) and Vactosertib combination in a first-ever such trial
- Manufacturing and quality release for CHM 2101 cell therapy's viral vector has been completed
- The company, which received over AU$3 million in R&D tax incentive, is undertaking a capital raising program to enable continued development of its cell therapy technology portfolio
Australian cell therapy company Chimeric Therapeutics (ASX: CHM) -- focused on discovery, development, and commercialisation of cell therapies to cure cancer – has been making significant strides towards its goal of bringing to life the promise of cell therapies for cancer patients. The recently released quarterly activities report for the period ended 31 March 2023 highlighted the R&D tax incentive refund and incremental progress on therapies including CHM 0201 and CHM 1101.
Chimeric has also received positive feedback for CHM 2101, a 3rd generation autologous CAR T cell therapy, from the US Food and Drug Administration during the pre-Investigational New Drug meeting. More details on major achievements during the quarter are below.
CHM 0201 - Phase 1B trial
In a first ever trial to evaluate the NK cells and Vactosertib combination on patients with advanced colorectal and blood cancers, the first patient was dosed with the combination in January this year. Notably, Chimeric's CHM 0201 (CORE NK) is said to be the best-in-class NK platform, with a previous study in a Phase 1 clinical trial demonstrating its safety (no GvHD, 28-day NK cell persistence, and positive efficacy signs in blood cancers).
The new Phase 1B study -- funded without financial support from Chimeric -- is centred on building further on the encouraging responses received in the Phase 1A trial by adding the oral TGF-β receptor inhibitor Vactosertib.
CHM 1101 - Phase 1A trial
Chimeric announced the start of treatment for first patient (glioblastoma) in cohort 4 in CHM 1101 Phase 1A clinical trial. The trial is ongoing at City of Hope, one of the biggest cancer R&D organisations in the US. Notably, this development follows the completion of CHM 1101 third cohort, in which no dose limiting toxicities were found.

Source: Company update
CHM 2101
Chimeric has achieved the manufacturing and quality release for the cell therapy's viral vector. This is being considered as milestone in the process of progressing the first in class, 3rd generation autologous CAR T cell therapy towards clinic. CHM is focusing on advancing this therapy towards trial in gastrointestinal and neuroendocrine tumours (Phase 1A).

Source: Company update
Positive pre-IND meeting
Chimeric has achieved positive conclusion of a pre-Investigational New Drug meeting (new CHM 2101 Phase 1 CAR T study) with the US FDA. The company has received encouraging written responses on the development plan for the cell therapy, which paves a clear way for the Investigational New Drug submission. During the meeting, the focus was on clinical development plan, technical operations, and other details.

Source: Company update
Subsequent to the progressive March 2023 quarter, Chimeric informed about the selection of CHM 1101 abstract for the 2023 Annual Meeting of the American Society of Clinical Oncology, to be held in June in Chicago, US. This follows the company's CEO and Managing Director Jennifer Chow presenting at the NWR Virtual Healthcare Conference in March 2023.
Lastly, one of the major financial highlights of the quarter was the over AU$3 million R&D tax refund received by Chimeric for its R&D activities during the last financial year.
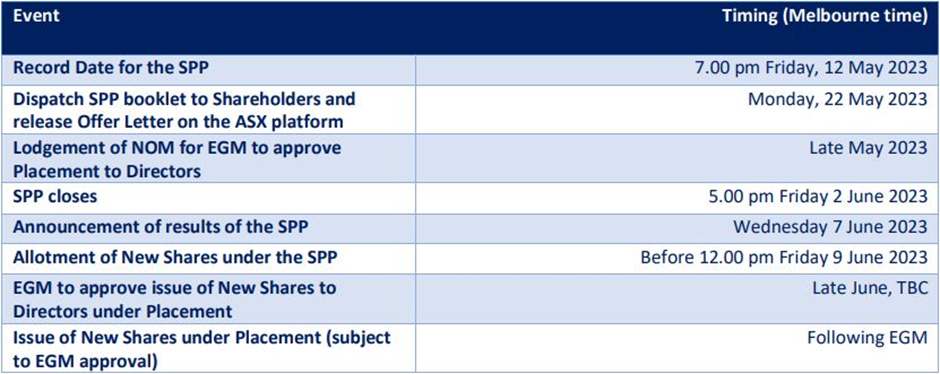
Subsequent to the reported quarter, Chimeric Therapeutics announced in May 2023 a share purchase plan (SPP) to raise up to AU$5.25 million, which accompanies AU$1.04 million raising via placement (commitments from CHM board and management). Read more. Chimeric’s CLTX CAR technology was granted patent in India and issued notice of allowance in Israel in April 2023. Read more.
Source: Company update