Highlights:
- Prescient is developing a portfolio of targeted therapies and cell therapies for cancer treatment.
- In September, the company joined hands with MD Anderson Cancer Center.
- The company believes CellPryme is now ready for clinical use and third-party incorporation.
- PTX-100 is thought to be the only geranylgeranyl transferase-1 (GGT-1) inhibitor in clinical development globally.
- Currently, the company is studying PTX-200 for relapsed and refractory acute myeloid leukemia.
Clinical-stage oncology company Prescient Therapeutics (ASX:PTX) is focused on developing its innovative pipeline in personalised medicine to address significant unmet needs. The company’s development portfolio comprises cell therapies and targeted therapies for a range of cancers. The company has licenced technologies and entered collaboration with renowned cancer centres in the US and Australia.
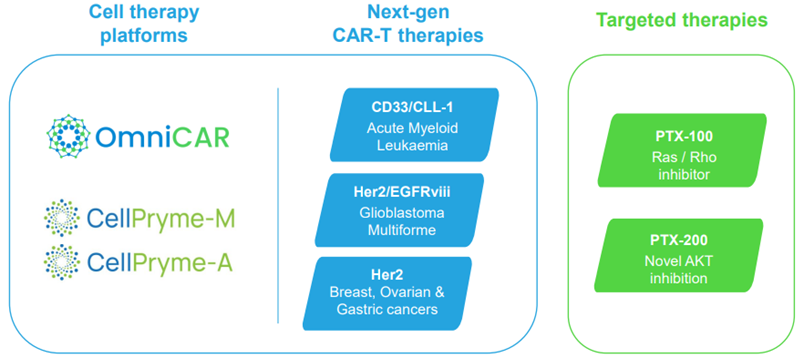
Image source: Company update
Image description: Oncology assets of PTX
OmniCAR
OmniCAR is a universal immune receptor platform enabling controllable T-cell activity and multi-antigen targeting with a single cell product.
The company considers OmniCAR as a transformational platform for cellular immunotherapy.
In September, Prescient entered a strategic collaboration with MD Anderson Cancer Center with the goal of developing best-in-class, adaptable CAR-T cell therapies to treat haematological malignancies. MD Anderson is the largest cancer centre in the US.
The partners will test the potential of this promising TCR-like binder for blood cancers. Read more here
CellPryme-M
CellPryme-M is a novel platform designed to enhance cell therapies. The platform produces superior cells that are long lasting and with higher tumour trafficking and penetration activities.
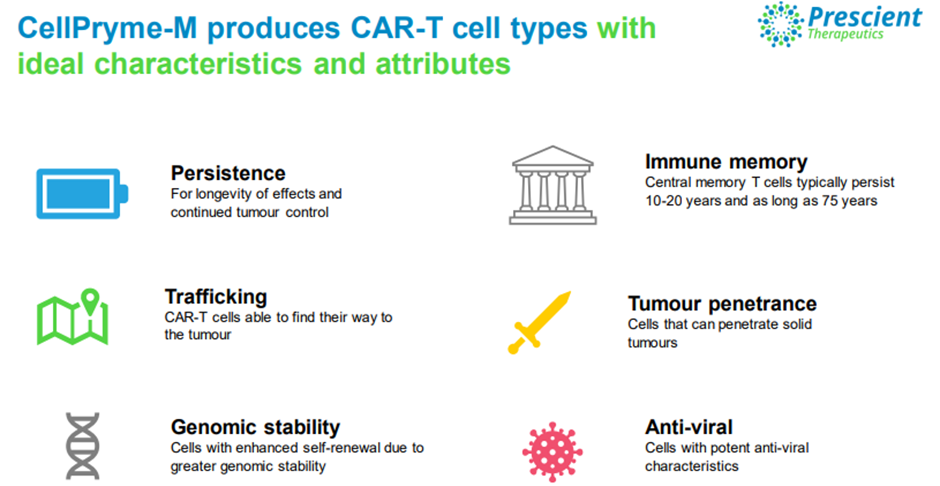
Image source: Company update
CellPryme-M has been manufactured at GMP grade and is ready for clinical use. As per PTX, the platform can integrate seamlessly into standard third-party programs and has revenue potential. It is also an attractive option for improving existing suboptimal CAR T products.
CellPryme-A
CellPryme-A is an adjuvant therapy that enhances tumour killing and hosts survival of conventional CAR-T cell therapies.
It overcomes the tumour’s hostile microenvironment and substantially improves the expansion CAR-T cells within the host.
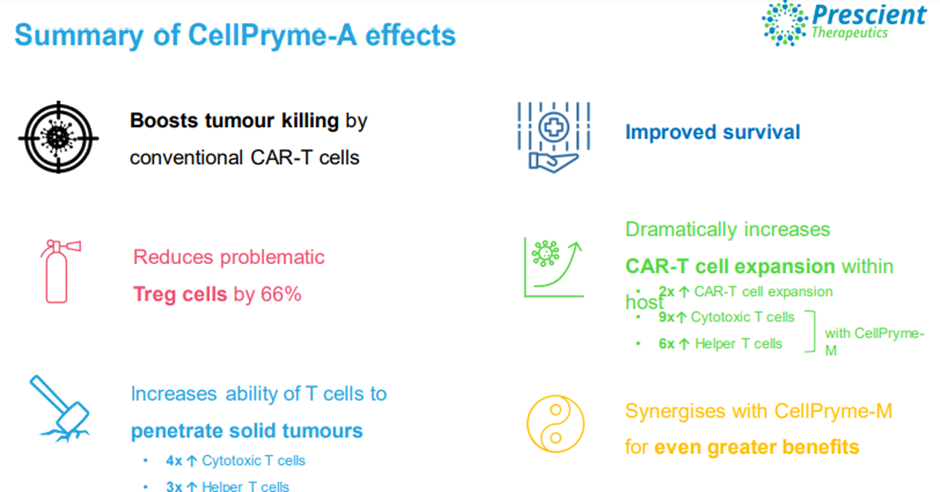
Image source: Company update
The company highlights that clinical trial-ready compound is also open for third-party collaboration.
PTX-100
The first-in-class compound licenced from Yale University, PTX-100 is believed to hold potential to block GGT-1, a key cancer growth enzyme.
The compound, which has received Orphan Drug Designation by the US FDA for Peripheral t-cell lymphoma (TCL), is thought to be the only GGT-1 inhibitor in clinical development globally.

© 2022 Kalkine Media®, Data and image source: Company update
In Phase 1b expansion cohort in TCL, PTX-100 has demonstrated a favourable safety profile compared to its peers. Currently approved drugs for PTCL have troublesome safety profiles; however, there are no serious impacts related to PTX-100. It suits the fragile patient population and is a good candidate for combination therapy, highlights the company.
Backed by these developments, Prescient has revised the study protocol with the addition of more patients with peripheral t-cell lymphoma.
PTX-200
A novel PH domain inhibitor, PTX-200 inhibits Akt, a critical tumour survival pathway. Akt plays a major role in the development of leukemia, breast, ovarian and other cancers.
The highly promising compound, which has secured Orphan Drug Designation from the US FDA, has a novel mechanism of action that inhibits Akt without non-specific kinase inhibition effects, thus differentiating it from other drug candidates.
Currently, the company is studying PTX-200 for the treatment of relapsed and refractory acute myeloid leukemia in a Phase 1b/2 trial. The trial has resulted in four complete remissions so far. It is currently treating the expansion cohort at 45 mg/m2.

© 2022 Kalkine Media®, Data and image source: Company update
PTX has witnessed significant progress in the last 12 months including
- achieving excellent safety and efficacy data from PTX-100 and PTX-200
- securing US FDA Orphan Drug Designation
- developing robust and reliable manufacturing and supply agreements
- entering important strategic and commercial partnerships
The company believes that these achievements cement its position as an emerging global leader, moving CAR-T therapies beyond their current limitations.
At the time of writing this article, PTX shares were trading at AU$0.150 midday on 5 December 2022.


