Definition
Related Definitions
Hydrocarbons
What are Hydrocarbons?

Source: © Aneese | Megapixl.com
Hydrocarbon is an organic chemical compound that is made up of carbon and hydrogen atoms. The carbon and hydrogen atoms are linked together in different patterns and configurations. These are found naturally in the form of coal, natural gas, crude oil and other energy sources. Hydrocarbons are highly combustible and produce water, heat and carbon dioxide on combustion. It is an effective and widely used source of energy. Hydrocarbons are also used as lubricants and as raw material for the production of various petroleum products, including fibres, plastics, explosives, solvents, rubber etc.
What are the different types of Hydrocarbons?
Copyright © 2021 Kalkine Media Pty Ltd.
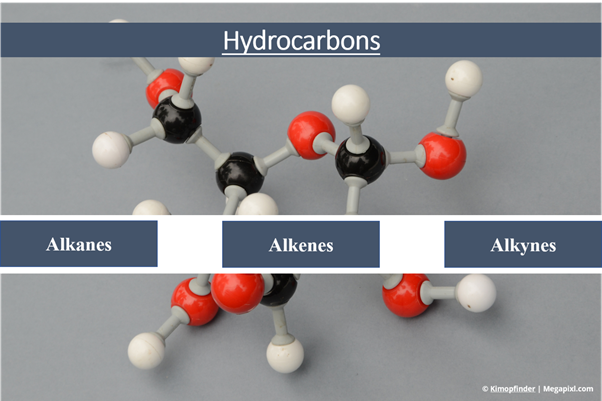
Hydrocarbons are classified as aromatic or aliphatic based on their sources and properties. The breakdown of oils and fats forms aliphatic hydrocarbons. They can be classified into alkanes, alkenes and alkynes. Alkanes contain single bonds; alkenes contain a double carbon-carbon bond whereas, alkynes contain a triple carbon-carbon bond. At the same time, aromatic hydrocarbons are made up of a group of related substances which are formed by the extracts of plants. Aromatic hydrocarbons can be further classified as arenes containing benzene rings or nonbenzenoid aromatic hydrocarbons that don't contain a benzene ring.
Where are Hydrocarbons found?
Almost all hydrocarbons are found naturally in the form of petroleum and natural gas. The decomposition of plants and animals over long time and under pressure lead to the formation of hydrocarbons, it is found deep into the earth deposited in underground reservoirs, just like water-soaked in a sponge. The reservoir rocks that are present below the earth's surface at substantial depth contains void spaces, and hydrocarbon is found stored in these void spaces. A well is drilled up to the depth of the reservoir to extract hydrocarbons from the subsurface to the surface. Both oil and gas are found deposited in these underground reservoirs.
How are Hydrocarbons formed?
Hydrocarbons are formed by the decay of organic matter. The organic matter gets deposited in a marine environment and remains buried for 100-400 million years. Over the period of time, the sediments overlap the organic matter layer by layer, and the organic matter subsides. As the organic matter subsides, it comes in contact with high pressure and temperature condition because as we go deep inside the earth, pressure and temperature rise. With increased pressure and temperature effect, the organic matter starts transforming into hydrocarbons slowly. Plate tectonics also play a vital role in the placement of reservoirs as rearrangements of the ocean and continents take place over this huge span of time. As a result of this, we encounter hydrocarbon reservoirs in both offshore and onshore locations.
Summary
- Hydrocarbons are organic chemical compound made up of carbon and hydrogen atoms linked together in different patterns and configurations.
- All hydrocarbons are found naturally in underground reservoirs in the form of crude oil, natural gas and many other forms.
- Hydrocarbons are formed by the decay of organic matter which comes from plant and animals.
Frequently Asked Questions (FAQs):
What are the different uses of Hydrocarbons?
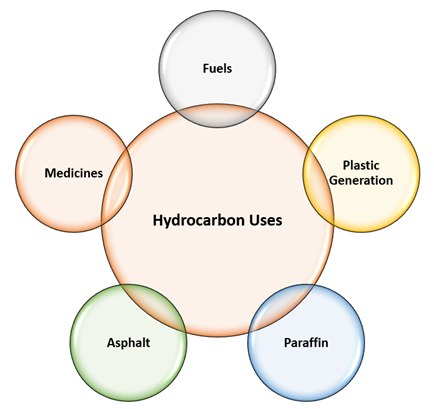
There are numerous applications of hydrocarbons. Let us discuss some of the most prominent uses of hydrocarbons briefly.
Copyright © 2021 Kalkine Media Pty Ltd
- Fuel: Most of the fuels we use in our day-to-day life such as Coal, LPG, LNG, Petrol, Diesel, Natural Gas, CNG are some sort of hydrocarbons.
- Plastics: Plastic is a long chain of monomer unit formed with the help of petrochemical products. Plastic is an integral part of our daily activity, starting from morning toothbrush to food containers, gadgets, car accessories, etc.
- Paraffin: This is used for medical purposes and for the making of candle.
- Asphalt: The heating of asphalt converts it into tar, an industrial product and widely used in road construction and other industrial applications.
- Medicines: Hydrocarbons are widely used in the manufacturing of drugs and pharms applications.
What are the concerns associated with Hydrocarbons?
Hydrocarbons are the main components of natural gas and crude oil. The burning of hydrocarbons releases an enormous amount of carbon dioxide into the atmosphere. Since carbon dioxide is a greenhouse gas, it is potentially responsible for global warming and a rise in global temperature. There are various ill effects associated with global warming and global temperature rise. An imbalance in carbon dioxide concentration can disturb the earth’s ecosystem and threaten the lives of living beings.
What are the alternatives to Hydrocarbons?
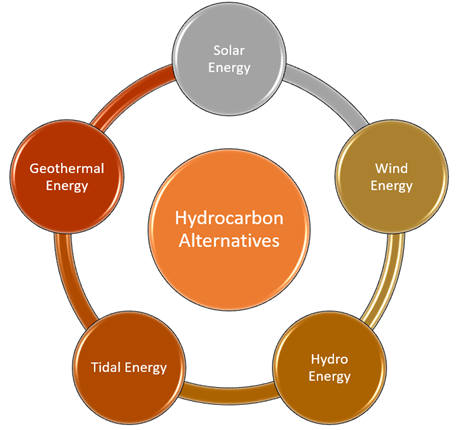
There are numerous alternatives to hydrocarbons that can replace them and help the world to supply an equivalent amount of energy without creating an imbalance in the earth. Let us discuss some of the prominent sources of alternative sources of energy.
Copyright © 2021 Kalkine Media Pty Ltd
- Solar Energy: This is a form of energy that is found in the Sun rays/light. Solar panels containing solar cells are used to convert solar energy into electric energy. The energy is stored in batteries and can be used later as per one's requirement. Solar energy has become a very popular source of energy in recent years.
- Wind Energy: This is another renewable energy source that is considered green or eco-friendly. Huge wind turbines are used to convert wind energy into electrical energy, which can be further used in a variety of applications ranging from household activities to industrial purposes.
- Hydro Energy: This is a form of energy that is derived from water. Water dams are constructed to store a huge amount of water in artificial reservoirs. A controlled flow from these reservoirs can rotate the turbines to create electricity.
- Tidal Energy: It is also a form of renewable energy which uses tidal energy to convert it into electrical energy. The high energy of tides is used to rotate the turbines, thus generating electricity. However, the tidal energy generation process can't be executed during low tides condition.
- Geothermal Energy: This is a form of energy that utilises natural heat energy present below the surface of the earth. The energy can be directly used for heating purposes in cold areas or used for the generation of electricity.
